Chromatography methods are used to help us separate molecular mixtures based on their mobility. One of the most basic versions of chromatography is thin layer chromatography (TLC). TLC can be used to separate chemical compounds and mixtures, identify specific compounds, and determine the purity of a compound.
Quick Overview
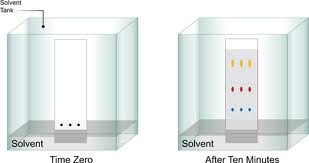
While there are many different types of chromatography, they all have two components in common: the stationary phase and the mobile phase. The stationary phase is fixed, while the mobile phase is able to migrate or carry substances through the stationary phase. The mobile phase is also known as the solvent. The stationary phase is generally a piece of glass, plastic, or aluminum that is coated in a very thin layer of an adsorbent material like silica gel. This thin coat of adsorbent material allows the mobile phase to separate the compound mixtures. The migration of the mixtures depends heavily on the polarities of the TLC plate, the solvent, and the mixture itself. The TLC plate tends to be of a higher polarity, while the solvent and mixtures are of varying polarity. When the TLC plate comes into contact with the solvent, mixtures are able to migrate through capillary action. After the samples have migrated on the plate, results can be visualized and quantified. Visualization can occur through ultraviolet light exposure, staining methods, or color observations. A compound’s movement can be quantified by measuring the retention factor or Rf value.
The polarity of the compound will determine the nature of its mobility on the plate. If a mixture contains two different components, the more polar component will adhere more to the plate and will migrate less. The less polar component will be able to migrate more quickly and further up the plate. The polarity of the solvent will also contribute to how fast and how far each compound will migrate. After your TLC plate has finished running, different spots will be visible. Each spot represents a different component within the mixture. After using one of the three visualization methods mentioned earlier, you can analyze the sample quantitatively by measuring the Rf value. Dividing the distance in centimeters (cm) traveled by each spot by the total distance traveled by the solvent will give you the Rf value of each component.
General TLC Procedure
- Cut a small piece of a TLC plate
- Generally, pieces are quite small (5 x 5 cm for example). Length of the plate depends on the molecular mixture you are analyzing. Some samples may require more space to separate.
- Draw a thin pencil line on TLC plate
- Draw a thin pencil line across the plate, about 1 cm from the bottom
- Spot samples on TLC plate
- Using a micropipette or a transfer pipette, spot a small amount of sample on the line drawn in step 2. Try to keep sample spots as small as possible. If there are multiple samples, make sure to space them out accordingly on the line.
- Fill your glass beaker with your desired solvent.
- Keep the solvent level in the beaker below the sample spots on the TLC plate.
- Run the plate
- Gently place the spotted TLC plate in the beaker with the solvent. Stand the plate straight up using the wall as a support.
- Remove plate from solvent
- Remove the plate from the solvent before it reaches the top of the plate. Draw a line where the solvent traveled to. This will be your solvent front, and will be used in your retention factor calculation.
- Visualize molecular mixture separation and analyze
- Using your desired visualization method observe the compound separation. Quantify your results by measuring the Rf of each sample.
Interested in learning more about TLC? Check out the following EDVO-Kits to learn more!
Dangerous or Delicious: Using Chromatography to Examine Vaping
Principles of Thin Layer Chromatographyhttps://www.edvotek.com/113