Getting ready for an epic semester of experiments? Don’t overlook (or avoid) the importance of a little pre-semester equipment TLC. We promise to be right there with you and make it effective and fun.
Let’s kick things off with the MVP of most labs—the mighty micropipette! These handheld wonders of liquid measurements are like reliable sidekicks, faithfully accompanying us through countless experiments. While they may sometimes feel like natural extensions of our hands during lab sessions, don’t be fooled—they’re powerful technological beasts that require care and maintenance. But fear not! Keeping these marvels in top shape is a breeze with our 4 C’s: Clean, Calibrate, Correct, and (use) Common Sense.
Clean
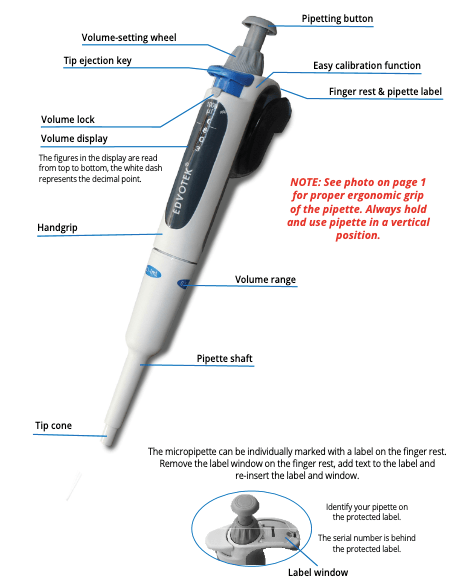
Keeping your pipettes clean is not just about appearances; it’s crucial for maintaining accuracy and prolonging their lifespan. During the semester, a simple wipe down with isopropyl alcohol or 70% ethanol at the end of each day or after every experiment should do the trick. Additionally, it’s a good idea to give them a more thorough clean once a year. While specific cleaning instructions may vary for each pipette, here’s a general guide to follow. Start by wetting a lint-free tissue or soft cloth with isopropyl alcohol. Then systematically wipe down all external surfaces, including the pipetting push button, the ejector push button, the hand grip, the body, the tip, and the bottom opening. If you encounter a stubborn and sticky substance, it’s generally safe to use a soft cloth dipped in a highly diluted soap solution, followed by another cloth with water for gentle scrubbing.
Dealing with contamination? Most pipettes can undergo sterilization as a whole in an autoclave at a temperature of 121°C for 20 minutes. After sterilization, ensure the pipette is dried and cooled down to room temperature.
That’s it, you’ve mastered cleaning! With newfound confidence, take a deep breath and embrace the next essential step, which we acknowledge can be intimidating at times: Calibration.
Calibrate
You might have heard that it’s essential to check pipette calibration regularly, ideally every few months and at least once a year, to ensure accurate dispensing of volumes. Perhaps you’ve been hesitant to tackle this task. But fear not, calibration is not as daunting as it may seem, and it’s a worthwhile investment for experimental success. So, let’s dive into the simple and easy steps involved in pipette calibration.
Here’s what you’ll need: water, a stack of pipettes, pipette tips, paper, pen, weight boats, and a quality analytical balance.
“But why a balance?” you may wonder. Well, water at a temperature of 20°C and under one atmosphere of pressure has a density of 1 g/mL. This fascinating property allows you to check the dispensing volumes of all your pipettes by simply pipetting some specific volumes and then measuring their weights.
Here’s a step by step:
- Fill a beaker with distilled water and let it sit for 15 minutes to reach room temperature.
- Check the temperature. If it’s 20°C, you’re good to go! If it’s slightly above or below, don’t worry. Water density changes predictably with temperature. Use this table to find the corresponding Z factor. Instead of multiplying your volume by 1 in step 5, multiply it by this number .
- Place a weight boat on a balance, close the door, and zero the balance
- Grab your first pipette, add a tip, and start pipetting (steps 5-9)!
- For utmost accuracy, pre-rinse the tip by immersing it about 2 mm in the liquid. Aspirate and dispense the set volume* three times, fully pushing when dispensing each time.
- Aspirate the liquid volume again and dispense it into a weight boat. This is the time to practice your finest pipetting techniques. When picking up the water, release the plunger slowly, wait for about 1 second after the water has reached the top before removing the tip, and even take the time to wipe away any drops of water from the outside of the tip. When dispensing also maintain a slow and steady pace.
- Place the weight boat on the balance.
- Record the weight on a piece of paper.
- Repeat steps 6, 7, and 8 ten times.
- Time to calculate (step 10-12)! Use the equation Volume = Weight of Water x Z factor to calculate the volume dispensed each time. Write your results next to the weights recorded in step 5.
- Calculate the mean volume from the ten trials.
- Determine accuracy using the following equation: Accuracy = 100 x Mean Calculated Volume (step 5b) / Nominal Volume (the intended volume you were trying to dispense).
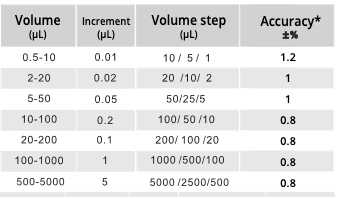
- Make a decision. Each lab may have its own specific accuracy targets, but for most molecular experiments, the table below serves as a reliable rule of thumb.
- Repeat steps 3-13 for the next pipette.
*But what volumes should I use? It depends on the pipette range. But a general rule is to test at the maximum volume, minimum volume, and a mid-volume. See the table above.
Correct
If your pipette fails the accuracy assessment, it will require recalibration. The process for recalibrating each pipette may vary, so it’s best to refer to the manufacturer’s manual for specific instructions. If you’re using Edvotek’s pipettes, you can find a simple six-step guide on how to adjust them by clicking here. Additionally, many companies and manufacturers offer services for calibrating and adjusting pipettes.
Common Sense
Now that you’ve got squeaky-clean and finely-tuned pipettes, preserve their accuracy throughout the year by following these four tips.
- Master proper pipetting technique: Practicing proper pipetting technique not only preserves the lifespan of your pipettes but also enhances the success of your experiments.
- Store your pipette vertically using a pipette holder: Storing your pipette vertically prevents any liquids in the barrel from reaching further inside.
- Avoid placing your pipette on its side when there is liquid in the tip.
- Adhere to recommended volumes. It’s crucial not to exceed the recommended volumes of your pipette. Operating within the specified range ensures accurate and reliable measurements.
Final Thoughts
Hard press amongst all the pre-semester to does to find time for pipette TLC? How about assigning lab groups the thrilling task of cleaning and calibrating their very own pipettes? Okay, maybe it’s not the most heart-pounding activity, but thre are undeniable benifits! Not only does this exercise introduce or reinforce essential skills like pipetting, measuring, and calculating, but it also instills a sense of ownership in their equipment. And guess what? That sense of ownership leads to better lab practices all year round!
Title image attribution: Magnus Manske, CC BY-SA 3.0 https://creativecommons.org/licenses/by-sa/3.0, via Wikimedia Commons


1 comment
Comments are closed.