In the coming weeks, the FDA is poised to greenlight a groundbreaking medical therapy. Exa-Cel is a revolutionary gene-editing treatment that treats sickle cell disease. Learn more about the first CRISPR therapy to seek FDA approval and then bring this exciting news – and the technology behind it – into your classroom!
What is Sickle Cell Disease?
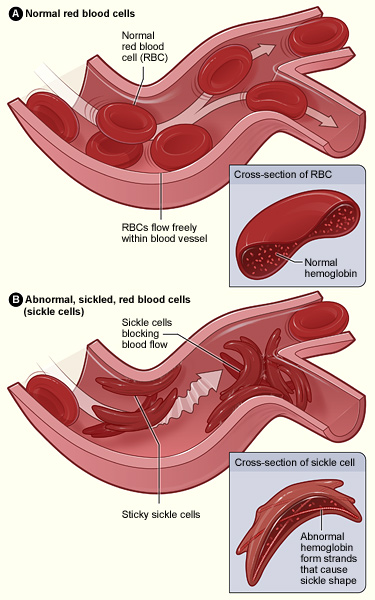
Sickle Cell Disease (SCD) is an inherited blood disorder caused by genetic mutations in the HBB gene. These mutations lead to the production of abnormal hemoglobin and result in a characteristic sickle shape of red blood cells. Individuals inherit SCD in an autosomal recessive manner, meaning both parents must carry at least one copy of the mutated HBB gene to pass the disease on to their children. When an individual inherits two copies of the mutated gene they develop SCD.
SCD’s symptoms vary in severity and can emerge at different life stages. In children, SCD may lead to delayed growth and development, jaundice, secondary infections like pneumonia, and episodes of excruciating pain called “pain crises.” Left untreated, these symptoms tend to worsen over time. In adults with severe SCD, chronic pain, fatigue, aseptic necrosis, vision loss, and life-threatening complications affecting the lungs and heart can occur, while milder cases may present with tiredness, dizziness, breathing difficulties, and a weakened immune system.
Over the last 50 years, there have been significant advancements in the treatment of SCD. In the 1970s life expectancy with SCD was less than 20 years old, while today people with SCD frequently live past 50. However, despite these improvements many with SCD continue to suffer from severe symptoms. Access to continuous and high-quality health care remains a challenge. Total healthcare costs for people with SCD reach $1 million by age 45, with annual costs around $10,000 for children and $30,000 for adults. Such care focuses on relieving symptoms and preventing complications but is unavailable on a reliable basis to many who suffer from the disease.
In some cases, a bone marrow transplant can cure the SCD, but such transplants are high risk, best done at a young age, and require a matched bone marrow donor. Consequently, the procedure is only recommended for children or teenagers with severe symptoms and/or dangerous complications.
The precise count of individuals affected by Sickle Cell Disease (SCD) in the United States remains uncertain but estimates hover around 100,000. Globally, SCD numbers are considerably greater due to its prevalence in regions heavily impacted by malaria, such as the tropical climates of Africa, the Middle East, and India. In addition, the incidence of Sickle Cell Trait (one normal hemoglobin gene and one sickle cell gene) is even more substantial, with certain populations reaching carrier rates as high as 1 in 10 individuals.
For more about Sickle Cell Disease check out our post on Sickle Cell Disease, the WHO’s SCD page, and the CDC’s SCD page.
What is CRISPR?
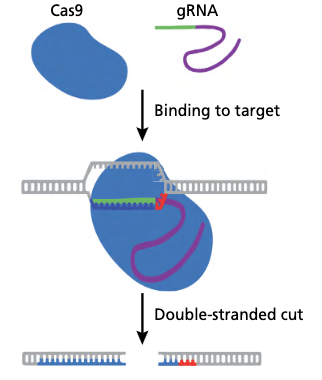
CRISPR, an acronym for Clustered Regularly Interspaced Short Palindromic Repeats, is a groundbreaking gene-editing technology that has revolutionized genetic research and biotechnology. It is a system originally found in bacteria as an immune response to viral attacks. At its core, the CRISPR system consists of two key components: (1) an enzyme called Cas and (2) a piece of RNA known as guide RNA (gRNA) or sometimes single guide RNA (sgRNA).
During a CRISPR experiment, the CAS-gRNA complex scans the surrounding DNA by unwinding short segments of DNA and compares them to the gRNA’s sequence. When the complex reaches a region of DNA that is complimentary to the gRNA sequence it attaches. The Cas enzyme then cuts the DNA. Once a CAS enzyme has unwound and cut a DNA segment the cell rushes to repair the damaged DNA. Depending on the specific repair process (predetermined by the experiment setup) this can either inactivate or “knock out” the gene or it can introduce new DNA nucleotides to the targeted region. CRISPR’s power is that allows scientists to precisely, accurately, and simply add, delete, or modify genes in most eukaryotic organisms.
For more on CRISPR check out our posts Biotechnology Basics: What is CRISPR? and Pack This Not That: CRISPR edition.
What is Exa-Cel?
Exa-cel, formerly known as CTX001, is a genetic therapy that uses CRISPR to knock out the BCL11A gene within soon-to-be red blood cells found in bone marrow. This gene (BCL11A) tells red blood cells to stop producing fetal hemoglobin after birth. This means that individuals who undergo Exa-cel begin producing unsickled fetal hemoglobin which crowds out the sickling “adult” beta hemoglobin. While there are differences between fetal and adult hemoglobin, this production still reduces many of the symptoms of SCD.
The Exa-cel treatment process involves several steps. Initially, stem cells, which are the precursors to red blood cells, are collected from the patient. Scientists then use CRISPR to disable the BCL11A gene in these cells. Next, the patient’s original stem cells are eliminated through chemotherapy. Finally, the CRISPR treated stem cells are reintroduced into the patient’s system via a transfusion.
Data from the first clinical trial for the treatment was presented to the FDA this fall. Thirty-one patients with severe SCD aged 12 to 35 underwent the Exa-cel treatment. Treatments were staggered so the observation period to observe the treatments efficiency and possible side effects ranges considerably from 2 to 32 months. However, at the time of reporting no patient had experienced a severe pain crisis and there was no evidence of “off-target” effects.
For a very personal and powerful account of the treatment, consider exploring NPR’s coverage of the first patient to ever receive Exa-cel therapy Victoria Gray here (3 minute listen) and here (4 minute listen). To gather further information about Exa-cel, visit the websites of the two companies behind this groundbreaking therapy – Vertex and and CRISPR Therapeutics.
The FDA Approval Process
If the FDA approves Exa-cel gene therapy for sickle cell disease, it could lead to dramatic improvements in the lives for many with sickle cell disease. Exa-cel would also become the first FDA-approved CRISPR therapy for a genetic disease. This could have wide-reaching impacts on individuals with other genetic diseases like cystic fibrosis and Tay-Sachs disease. Because of this Exa-cel was given a Priority and Fast Track designation by the FDA at the start of the approval process.
In the second to last step of this process, a committee of independent FDA advisors met on October 31st, 2023 to make a formal recommendation based on their expertise and the initial clinical data. The committee reviewed and recognized the incredibly positive initial results. They also discussed several concerns surrounding side effects, unknowns, and accessibility.
Uncertainties around long term outcomes surround Exa-cel. Multiple years of follow-up are needed to prove that the treatment can reduce the organ damage associated with sickle cell disease. It’s also unknown whether stem cells treated with Exa-cel will continue to produce non-sickling red blood cells for the rest of the person’s life or if they will die off over a certain number of years. Because the treatment requires chemotherapy, there are also concerns about chemotherapy-associated complications, such as infertility or secondary cancer. Most importantly the panel examined the possibility of off-target effect – unintended genomic alterations that could have cascading effects.
Another major issue surrounding Exa-cel is access. A report from the nonprofit Institute for Clinical and Economic Review estimated the cost could be close to $2 million per patient. Even with FDA approval such a high-cost medication may come with insurance barriers and rules. Individuals may be ineligible because they have a different genotype, have suffered too much organ damage, are the wrong age, or for other non-biology-based reasons. Several sickle cell associations including the American Association of Sickle Cell have raised concerns that a portion of SCD suffers will not have access to the treatment and may still need more traditional treatments which they emphasize should remain a focus.
Considering both the treatment’s efficacy and the remaining concerns, the committee formally recommended FDA approval – provided that the companies continue to asses safety risks even post-approval. Lisa Lee, a bioethics expert from Virginia Tech University and panel member, said that the pivotal question posed was” “Are these uncertainties more detrimental than hindering the progression of this treatment?” and that the consensus was that they weren’t. Vertex has already pledged a 15-year follow-up on patients, not only from the initial trial but also in subsequent trials and any FDA-approved treatments.
The recommendation by the committee is a strong indication that the treatment will soon become available. However, the FDA still has its final decision on whether Exa-cel will be approved for sickle cell patients. This decision is set to be made on December 8.
Sharing current discoveries and breakthroughs can invigorate a classroom. Such news sparks engagement and cultivates a tangible understanding of biology’s impact. If you’re incorporating hands-on experiments into your classroom this year, consider delving into the world of sickle cell disease or introducing the fundamentals of CRISPR. Enhance the experiment by weaving in this unfolding and historic news, providing students with a direct connection to the cutting-edge medical advancements that are improving lives and shaping the future.



2 comments
Comments are closed.