Chromatography is a fundamental technique in biochemistry for separating, identifying, and isolating molecules from complex mixtures. Through the exploitation of physical or chemical differences between base components such as size, charge, hydrophobicity, or specific binding affinities, chromatography allows scientists to isolate proteins, nucleic acids, lipids, and small metabolites with precision. There are several types of chromatography, each designed to separate a particular type of molecule and set of conditions.
Column chromatography is a general method for the separation of biomolecules based on their interaction with a stationary phase fixed within a cylindrical column. The stationary phase is usually a collection of solid beads suspended in a solvent. The sample is loaded to the top of the column and a solvent (mobile phase) is added to pass the sample through. Different components within the sample travel at different speeds based on their interaction with the stationary phase. This technique is versatile and widely used for protein purification in laboratory settings.
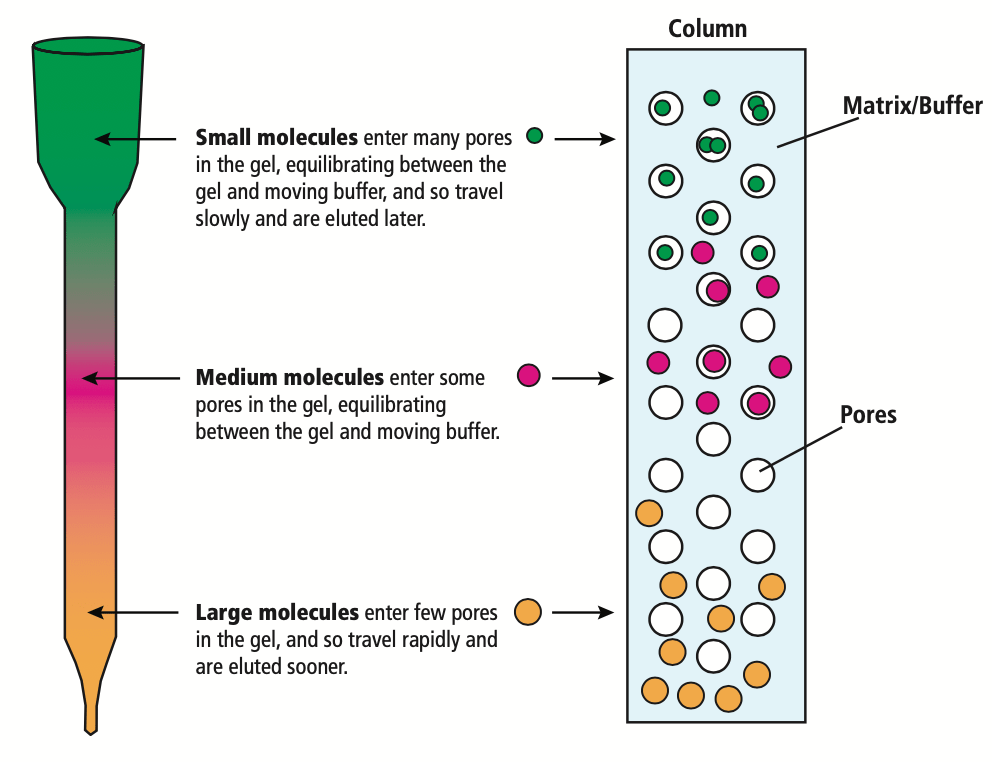
Size Exclusion Chromatography (SEC)
Size exclusion chromatography, or gel filtration, is a size-based separation method. Porous beads in the stationary phase allow smaller molecules to penetrate and move slowly down the column, while larger molecules are excluded and pass through the column first. This method is typically utilized for molecular weight estimation or removal of trace impurities like salts from protein solutions. For anyone wanting hands on experience with size exclusion chromatography, check out Edvo-Kit 108!
Ion Exchange Chromatography
Ion exchange chromatography separates molecules based on their net charge. The stationary phase consists of charged resin beads, either positive (anion exchange) or negative (cation exchange) charge. Biomolecules from the sample are drawn to the resin based on their charge, and then eluted with a salt or pH gradient. This technique is extremely valuable in the separation of proteins or nucleic acids with similar molecular weights but distinct charge characteristics.
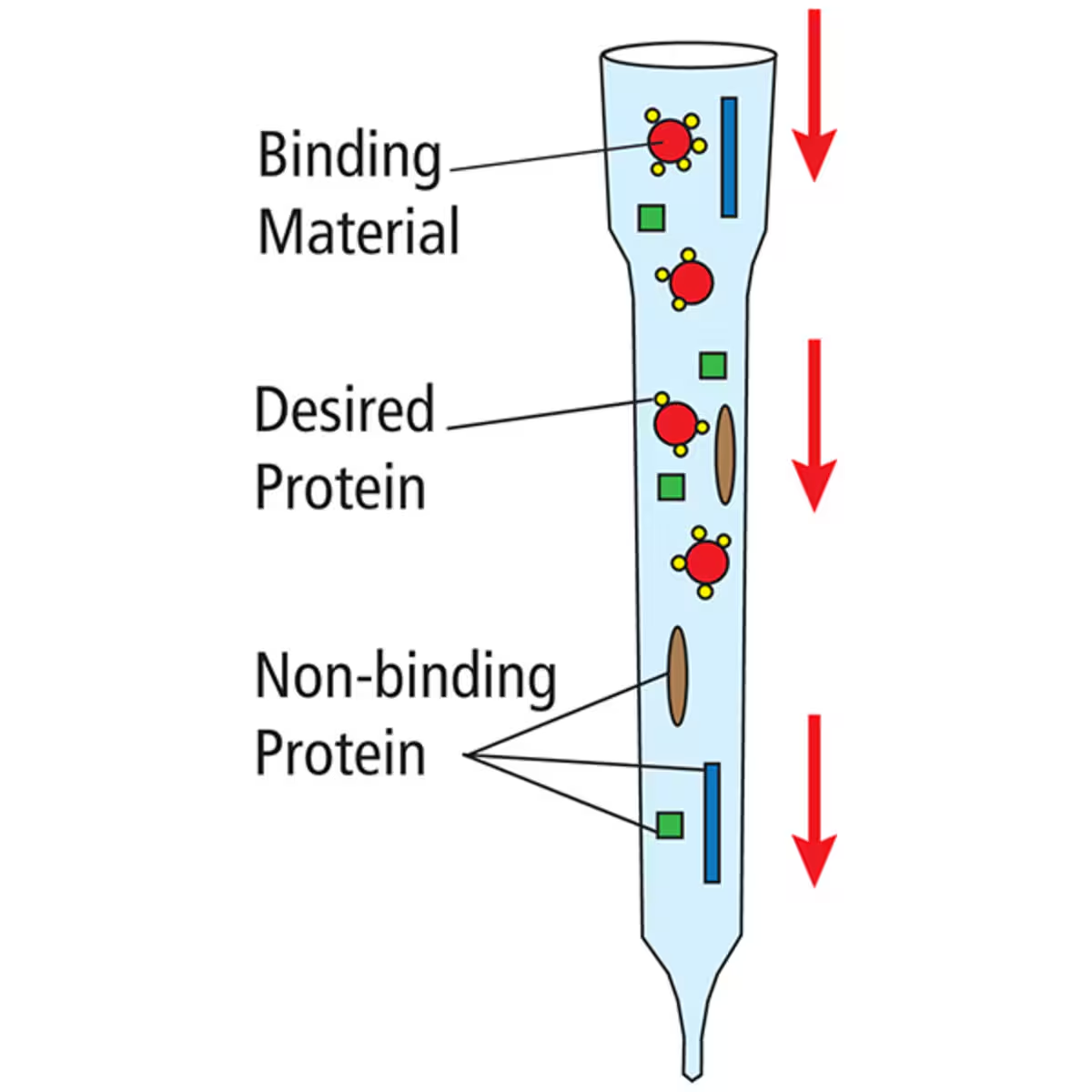
Affinity Chromatography
Affinity chromatography is a highly specific method that relies on molecular interactions between the target molecule and a ligand attached to the stationary phase. Generally, the target molecule contains a certain motif that specifically binds to the stationary phase. An example would be a protein with a His-tag that will directly attach to a nickel resin, or an antibody that will be attached to its antigen. Following the removal of non-specific molecules by washing the column, the target is eluted with the aid of a competitive agent or through alteration of conditions such as pH. This method is widely used for the purification of specific enzymes and recombinant proteins.
Thin Layer Chromatography (TLC)
Thin-layer chromatography is a simple, fast technique used primarily for qualitative analysis. A tiny sample is applied to a plate containing a thin layer of adsorbent like silica gel. When the plate is put in a solvent, the sample compounds move with different speeds depending on their strengths towards the stationary and mobile phases. TLC is frequently used for monitoring a reaction’s progress or basic separation of lipids and small molecules.
High-Performance Liquid Chromatography (HPLC)
HPLC is an advanced and highly effective technique that provides high-resolution separation of biomolecules. HPLC employs the general method of column chromatography but utilizes high pressure pumps to precisely control the flow of mobile phase across the stationary phase. Reverse-phase HPLC, the most common type, separates molecules based on hydrophobicity using a non-polar stationary phase and a polar mobile phase. Normal-phase HPLC does so in the reverse manner. HPLC is commonly used for analyzing small biomolecules such as peptides, nucleotides, and metabolites with maximum precision and efficiency.

Chromatography provides a flexible set of tools for accurate separation and examination of mixtures. While there are many methods of chromatography, the general principle of passing a sample across a stationary phase in order to separate individual components remains the same. Each method of chromatography leverages various molecular attributes, so the appropriate method must be selected for a specific application. Additionally, different methods of chromatography are often performed sequentially to yield a very pure product. Purifying a protein, examination of a metabolite, or tracking a reaction’s progress are all situations in which chromatography is an invaluable technique in contemporary biochemistry. If you are interested in further exploring chromatography in the classroom, check out these kits below: